
The Battery Cycle #6: SOH… What is that?
Below is a new contribution from Claudius Jehle, CEO of volytica diagnostics GmbH. It’s the sixth of a series of knowledge articles on topics around Li-Ion Batteries.
What is the excessively used “SOH”? How is it defined, what is influencing it, how can it be determined?
Phenomenology – How degradation makes itself noticeable
As we explained in the last articles, the basic storage principle of Li ion batteries is that Li ions are embedded in a host electrode, from which they are re-extractable. A “full” battery has basically all seats of the anode occupied by ions1, while the cathode seats are empty. When discharging, the ions stand up, release an electron, migrate out of the respective electrode (which is comparably slow, given their bulkiness, as we learnt), swiftly swim the few micrometres through the electrolyte-infused separator, just to remigrate into the opposite electrode. The electrons take the long way through the vehicle or system electronics, powering them. All reunite on the other side.
We will dedicate a whole article on stress factors (“What Hurts?”) but for the moment believe us that this simple process has several “interfering” factors. For many chemistries, warm temperatures increase ion mobility – directly resulting in more ions per second throughput. That is: Lower resistance. In macroscopic terms: More power. Faster Charging. Faster Acceleration.
Nice! But also: Higher distraction for the ‘reaction-happy’ ions. The warmer, the higher their tendency to flirt with the myriads of chemical agents inside the battery. In such ‘side reactions’, ions combine with other components and are lost for energy storage. Also, if too cold, or if too many ions are congesting at the surfaces of the electrodes (i.e. too fast charging/discharging), or if the destination electrode is already too full, or dozens of other chemistry-depending combinations, ions get caught in unpleasant side reactions that remove them from service.
Voila: This loss of active material reduces the capacity and these useless side products clog the migration paths, increasing the internal resistance and lowering the power capabilities. That’s it.
Note: It will become very relevant later to understand that, unfortunately, there is no universal relation between “amount of capacity fade” to “amount of resistance increase”. In other words, while most of these side reactions (there are dozens, if not hundreds) result in some form of capacity fade or another, some result in considerable resistance increase, and some are not clogging at all.
We cannot continue without mentioning this: Some of those side reactions are subtle and: mean. They consume Li, but unlike the boring reactions, they result in sharp, acute and aggressive residues. Only when it’s too late and those defects grow too large and damage the battery internally, the effects become noticeable: Leakage or Fire. An own article will be dedicated to those hidden and obscure, highly safety-relevant topics of Li-Plating and Thermal Runaway.
But for the moment we limit ourselves to the less safety-relevant effects. Look at the following 15min excerpt of an e-bus driving profile2. The current profile (top), i.e. the demand of charge that a specific route demands from the vehicle, of course does basically not change over the years (unless the route changes). But the voltage or filling height does – significantly! The trained eye discerns the two above mentioned effects: (a) the battery generally depletes quicker as capacity fades and (b) the reactions to current jumps (braking/accelerating) kick higher, i.e. the voltage drops farther given the same current input3.
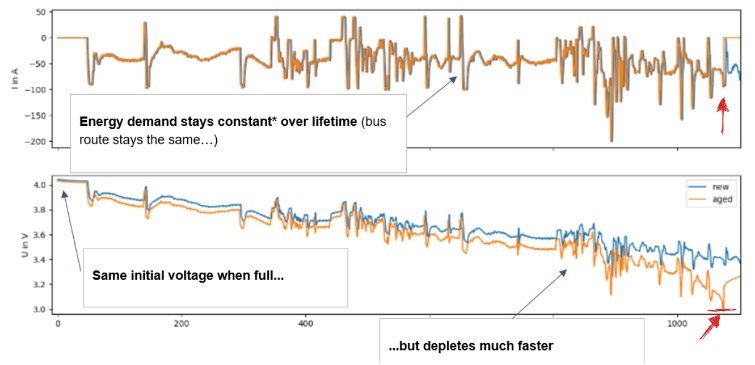
What’s happening at the end, to the very right? A minor pedal step (humble current jump in upper plot, red arrow) drags the already-low voltage below the absolute minimum admissible safety limit of 3.0V (lower plot, red arrow), leading the system to shut down (current jumps to 0, upper plot).
So: Degradation (the boring one) limits the asset’s fitness by reducing the operational degrees of freedom up to the point of shutdown. Capacity fade and resistance increase both affect voltage, intertwined and inseparable. A single performance indicator can thus not be sufficient to describe operational fitness, let alone safety.
State of Health, SoH or SOH
Yet there is one single very prominent parameter in which this hope and illusion is often projected – poor SOH is bearing this burden almost alone! What is SOH actually? Well, we don’t know for sure, it depends on the context and whom you ask. If stated without any circumstantial information, one can assume that the remaining capacity relative to the pristine capacity is meant (and e.g. not the resistance, the combination of both, safety, … or so).
Short recap from article #3: Current in/out-flow leads to ‘foam’4. A battery cannot be (dis)charged arbitrarily quickly. Inflow generates energy-free voltage components that force a current rate decrease once the energy-containing voltage plus this ‘foam’ reach the absolute maximal voltage. Just like drafting a glass of beer.
Given that effect, speaking about ‘the’ capacity of a battery (or a glass), in the very meaning of the word, is not possible. There is no “one” capacity. The amount of charge you get into a battery depends on the conditions. If done at high current, you get less in (too much foam). If it’s cold, you get even less in (even more foam). If you charge veeeery sloooow, no foam is building up, and you get much more in (cf. especially the so-called CV-phase at the end of charging in the charging article). If you charge non-constant (i.e. you use the thing) you get something in between. Of course, the said applies to discharge/usage likewise. That is crucial to understand.
Often, the “SOH” is thus implicitly understood as “The amount of charge dischargeable at laboratory conditions with a constant current of 1C until the voltage hits the abs. minimal threshold” (the “SOHc@1C”). Knowing this number tells you something like: “The range that an asset can travel at laboratory conditions if driving uninterruptedly at constant speed without stops.”. Often, it is also understood as the capacity at very low currents (the 0C capacity). This is good for cross-asset comparability, but often only theoretically helpful for daily operation.
Note: If one wanted to display a battery in a very optimistic light, one would unilaterally give the SOH as the chargeable capacity with a CCCV-profile at low current, which can be significantly higher than the inverse case. This is not academic quibble: The differences can be 5-30% of capacity!
Battery State of Health: Measurement and Certification
A direct consequence of this is that SOH is hardly measurable in that very sense. I am not referring to its undefinedness, we could agree on a definition. No – the conditions do not occur in daily business! Never is a bus driven at constant low speed until it stops in the middle of the road (and at lab temperature), to be towed back. So the onboard electronics are having a hard time to estimate residual capacity, aggravated by the problems of ‘foam’ and ‘glass shape’. Given the limited computational power and the dissimilarity between daily operation and lab conditions, any onboard-estimated residual capacity has to be treated with utmost care, caution and awareness.
Some words on ad-hoc or quick tests: We learned that capacity fade goes hand in hand with resistance increase. Thus, there are multiple efforts to circumvent the cumbersome and lengthy procedures by indirect measurements – compare the Covid quick test: the Lateral Flow Tests don’t check for viruses, but for indirect cues. Information on resistance – or more technically: impedance – can be measured (!) quickly, within seconds or minutes5. So if both processes occur alongside, there should be a relation? Quick resistance measurement, lookup table, capacity information. Look at the next figure – on the vertical axis, a resistance-related value is given. The 3 colored areas (I added the lightly colored areas to encircle the dots) contain information from 3 forms of capacity measurements (sidenote: you can see very nicely that depending on the testing procedure even in a lab the SOH results significantly differ!).
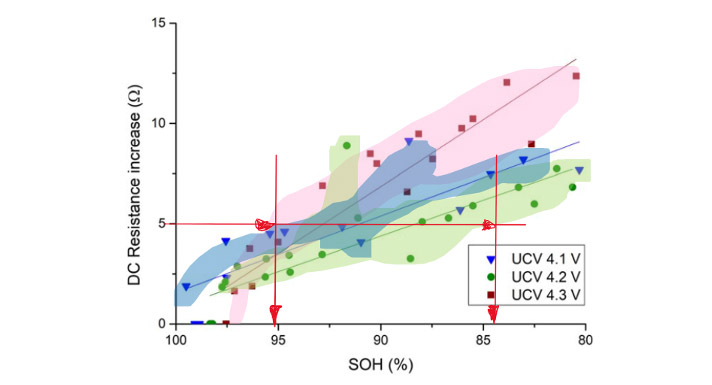
Say one measured 5 Ohms (red lines). The corresponding SOH could be anything from c. 85-95%. And that mustn’t surprise: I mentioned earlier that not all side reactions affect resistance increase as they affect capacity. So there must be a huge spread. You see what I mean: A resistance/impedance-based quick test is nothing but a quick test – it gives insights and hints, but cannot replace a thorough investigation. And no information on safety issues is contained.
A lot more information and value is contained in operational battery signals that have been transmitted via telematics into a server in the Cloud. Today, the transactional costs for transmission and storage are negligible and are eclipsed by the added value – warranty claims, replacement and purchasing decisions of batteries are inseparably tied to battery quality. We promote that more awareness is given and more care is taken when assessing an assets operational fitness, residual lifetime and/or value, and that the unilateral focus on “SOH” is at least questioned and challenged by the industry.
Outlook
In the next articles, we will cover the question what actually influences degradation (stress factors), we will cover the question of safety (other than operational fitness, as covered here), and meaningful End of Life definitions (“SOH=80%” – why?), as well as 2nd life and the like. Stay tuned.
Take home messages
- Degradation has only two directly noticeable effects: capacity fade and resistance increase
- The effects are inseparably intertwined. Operational fitness or residual value can thus not be represented by a single “SOH” value. SOH is more a concept than a number.
- There are also ‘hidden’ effects of degradation that unfortunately have an extreme safety relevance (see later articles) – but those don’t run under the concept of SOH!
- SOH is not a measure for safety. Again: SOH is not a measure for safety!
- Capacity fade, resistance increase, operational fitness, stress factors and all other aspects are very hard to measure by quick tests or electronics – don’t procrastinate to implement a proper data acquisition, analysis and asset management system
Footnotes:
1 Actually, they are not ions but atoms at this stage, but for the sake of simplicity we will not distinguish here. Bear with me!
2 The profile was recorded via telematics and subsequently replayed onto cells in a laboratory for several years. Note that in a real world application, the current profile would change. For sake of simplicity and demonstration, this is neglected here.
3 Okay we agree, this is difficult to discern; in fact, the orange jumps in voltage are c. 32% higher than the blue ones!
4 Disclaimer once again: not real foam, but a metaphor. Please read the other articles.
5 Using simple algorithms or additional sensors like EIS (electrochemical impedance spectroscopy)
6 Landa-Medrano et al., Journal of The Electrochemical Society 167/9, 2020, https://iopscience.iop.org/article/10.1149/1945-7111/ab8b99
Originally published on Sustainable Bus


